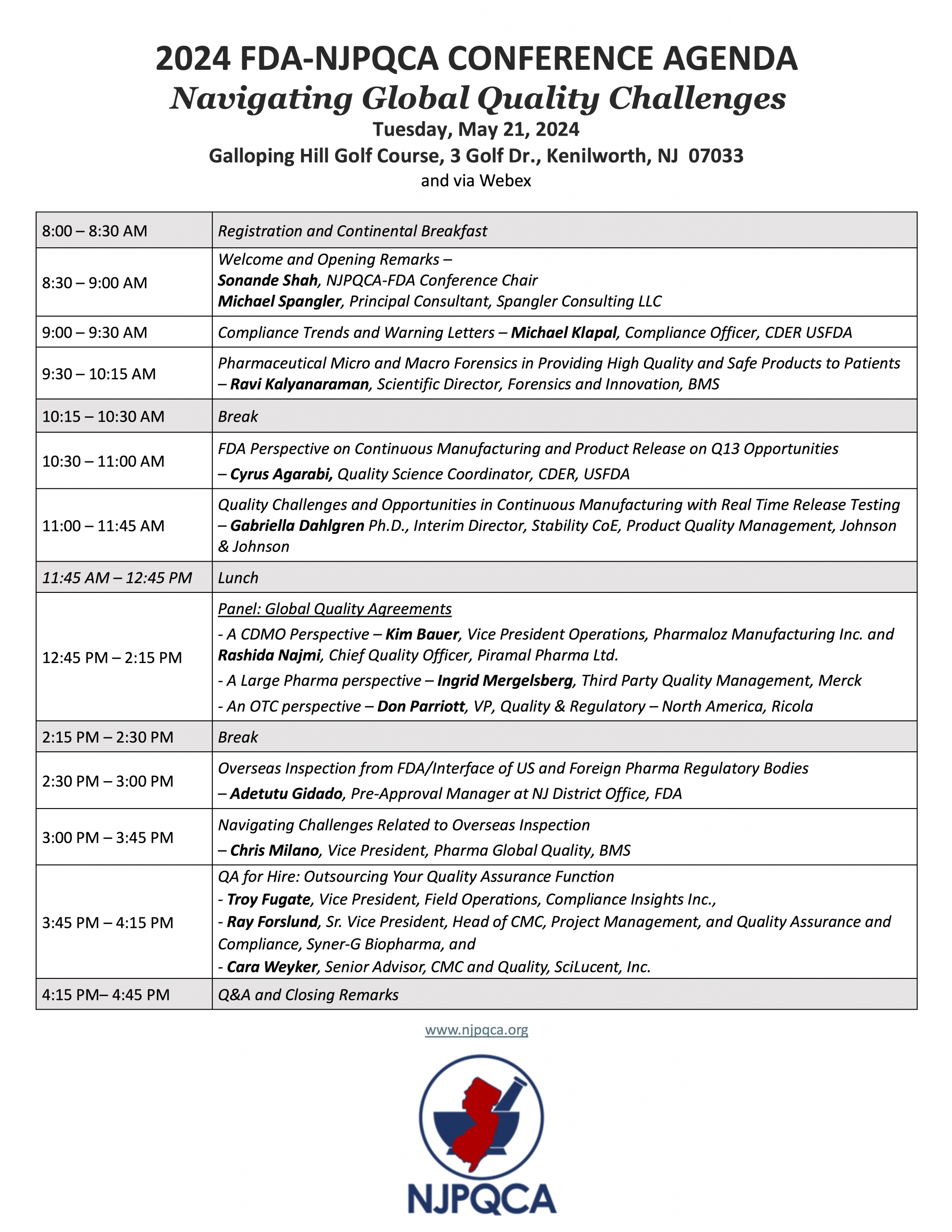
We are pleased to announce the 2024 NJPQCA-FDA Conference!
See below for updated Agenda and Flyers with FDA Speakers!
- Date: Tuesday, May 21st
- Location: Galloping Hill Golf Course in Kenilworth, NJ and online
- Theme: Navigating Global Quality Challenges
Join us…
- Gain the most current perspective on FDA expectations.
- Listen and learn from the senior leaders and SMEs from FDA, Bristol Myers Squibb, Johnson & Johnson, Compliance Insight Inc., Syner-G BioPharma, and SciLucent Inc. on compliance issues, continuous manufacturing and release, counterfeiting, outsourcing QA, and overseas inspections.
- Engage in a Panel Discussion on Global Quality Agreements with industry SMEs from Merck, Ricola, Pharmaloz Manufacturing Inc., and Piramal Pharma Ltd.
See below for more details about agenda and speakers.
Register at this Eventbrite link.
Note: This Conference will be available in a hybrid format (both in-person and online) to allow an opportunity for all to attend. The cost is the same for in-person and online attendees.
Members $300
Non-members $400 (Early Bird $350 by April 30, 2024)
Groups of 3 or more per company $350 each person
Government/Compendia Employees $150
Comments from our 2023 Conference participants:
The Panel Discussion on vendor quality was fabulous…loaded with information!
FDA’s retrospective and prospective presentations were most helpful
All topics were relevant, with great content
Learned from each presentation
Time and pace of presentations were strong, everything was excellent
Good format
Nice location
Keep up the good work for the industry!